Pharmaceutical products are usually packaged in containers for easy storage, distribution and use, and to protect the quality of the product. Information on the stability of the medicinal product in the selected container over the proposed shelf life is part of the quality information required to obtain regulatory approval for any medicinal product to market, in addition to evidence that the product is safe, effective, high quality and conforms to current good manufacturing practice. Product stability is usually demonstrated by conducting practical stability studies under ICH conditions. Due to the time-consuming nature, for long-term stability studies of pharmaceutical products, it is important to select the appropriate container to ensure the success of the study, especially where product packaging protection is required.
Product stability can be achieved through formulation design and proper use of containers. However, formulation design alone cannot ensure product stability during storage. Due to the diversity of pharmaceutical properties and their interactions with environmental conditions, some pharmaceutical products require protection against degrading factors such as humidity and oxygen. Therefore, selecting the proper packaging to provide the desired protection is an important part of product development. Although the selection process can be simplified by using the highest protective container closure system, the high cost may make it difficult to justify or practical for commercialization unless a high barrier container closure system is really required. Therefore, an appropriate container closure system must be selected to balance the need for product protection and the cost of commercialization.
Formal stability studies are the process of demonstrating the quality of a drug product over its shelf life in a selected container closure system. Formal stability studies are not the best way to select containers, as the process is empirical, time-consuming, expensive and inefficient. Proper selection of containers can be achieved by utilizing a science-based and quality-by-design (QbD) approach in the early development stages, before formal stability studies. The container can be selected based on material properties and relating the properties of the drug product to the properties of the container. Formal stability studies are then used to demonstrate the stability of the product in the selected container closure system.
This chapter will focus on the scientific selection of containers and/or packaging materials for solid oral dosage (SOD) to provide protection.
1. General considerations
Packaging materials used for pharmaceutical products must meet regulatory agency and pharmacopoeia requirements, provide adequate protection to the product from degradation and contaminating agents, perform their intended function properly, and be compatible and safe for use with the drug product. In addition, the packaging should be of a convenient and cost-effective nature for both the patient and the pharmaceutical manufacturer.
Containers are used for different types of products and different routes of administration. Different types of products have different container requirements. Container-drug interaction concerns are greatest for parenterals and inhalers, and lowest for solid dispersions. For detailed information on different types of drug products, please refer to FDA's guidance <Container Closure Systems for Packaging Human Drugs and Biologics>.
Proper use of container closure systems can protect SOD from moisture, light, oxygen or other gases, microbial contamination, and mechanical stress. In general, protection from moisture, light and oxygen are the most important factors. Control of microbial contamination can be easily achieved through the use of well-sealed containers and controlled materials, manufacturing and packaging processes under good manufacturing practice conditions. Protection from physical stress is achieved by using containers with adequate strength and minimized headspace to prevent excessive movement of product during handling and shipping. Containers generally do not protect the product from heat stress. For heat-sensitive products, it is usually protected by refrigeration or freezing during storage, transportation and use.
2. Container classification
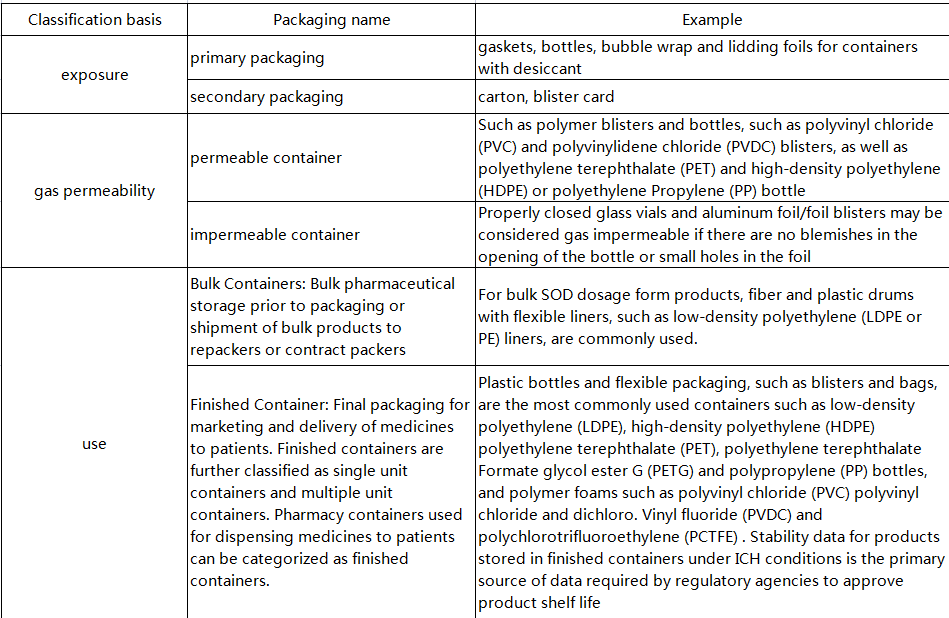
3. Desiccant
Desiccants are often used to keep products dry and stable. Desiccants can absorb moisture from the air through physical adsorption or chemical reaction, thereby reducing the humidity in sealed containers. Moisture absorption of silica gel is an example of physical adsorption, and moisture absorption of calcium oxide is an example of chemical reaction.
3.1 Classification of desiccants For solid pharmaceutical products, the most commonly used desiccants are silica gel, clay and molecular sieves.
3.1.1 Silica gel
Silica gel is amorphous silicon dioxide and is highly porous. Silica gel performs well at room temperature, but adsorption rates and equilibrium humidity may be reduced at higher temperatures. Moisture in silica gel can be removed by drying at greater than 110°C. The current USP Chapter 671 recommends pre-drying silica gel desiccants at about 150°C to ensure complete removal of adsorbed moisture.
3.1.2 Clay
Clay is a low cost desiccant that works well at low temperatures. The main chemical components of clay include silica, alumina, magnesia, calcium oxide and iron oxide. Different grades of clay desiccants have different moisture absorption capabilities. Clay works well at room temperature, but at temperatures below 50°C it may lose moisture instead of absorbing it.
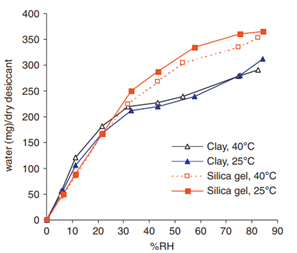
Equilibrium adsorption capacity of silica gel and clay
Humidity sorption isotherms for desiccants can be found in the literature. The equilibrium adsorption capacities of silica gel and clay at different temperatures and humidity are shown in Fig. 1. One caveat is that different grades of desiccant of the same type may have significantly different moisture adsorption capacities. Therefore, it is imperative to verify the humidity sorption isotherm of the chosen desiccant.
3.1.3 Molecular sieve
Molecular sieves are highly porous crystalline materials with precise monodisperse pores that can accommodate molecules of a specific size. Molecular sieves are available in different effective pore sizes such as 3, 4, 5 and 10. A molecular sieve with an effective pore size of 3 can selectively adsorb water molecules because the diameter of a water molecule is about 3, while a molecular sieve with a pore size of 4 can also adsorb oxygen in addition to water molecules.
3.2 Desiccant Dosage It is important to consider the amount of desiccant used, an insufficient amount will not provide the desired protective effect, while too much may lead to overdrying and increased costs. In many cases, overuse of desiccants does not affect drug product quality. However, excessive drying of some hydrates may lead to unstable amorphous formation. Therefore, it is very important to understand the product characteristics, the degradation mechanism of the product, and the humidity range required by the product before determining the amount of desiccant.
3.3 Effectiveness of the desiccant Because when exposed to air, the desiccant may rapidly absorb water vapor, thereby reducing or even losing its protective ability. In order to maintain the effectiveness of the desiccant, the environmental conditions and packaging process in which it is placed in the product container must be well controlled, and the ambient humidity and exposure time in which the desiccant is placed in the container must be clearly reduced if necessary.
4. Oxygen absorber
When the product is sensitive to oxygen in the air, the following methods can be selected for protection.
1. Use antioxidants
2. Pack the product in a container impermeable to oxygen, such as a glass or aluminum container, combined with an inert gas such as nitrogen or argon.
3. Synergistic protection of medicines through formulation design and packaging
Normal air contains about 21% oxygen. Oxygen levels can be reduced to a range of about 0.5-5% with inert gases. Oxygen levels in sealed containers can be reduced to less than 0.1% with adequate quantities of oxygen absorbers. Reducing initial oxygen levels combined with the use of containers with low oxygen permeability supplemented by oxygen absorbers may be the best method of protection for oxygen sensitive products. An oxygen absorber is a material that reacts with oxygen. Oxygen absorbers are common in the food industry but are less commonly used in pharmaceutical products. Different types of materials used as oxygen absorbers include:
Inorganic oxygen absorbers, such as powdered metals or their intermediate oxides;
organic antioxidants, such as vitamin C or salicylates;
Polymer oxygen absorber.
Unlike desiccants, oxygen absorbers have not been well studied for pharmaceuticals. Therefore, extensive research is required, including material properties and characterization of the redox mechanisms of oxygen absorbers and drug products, to evaluate the protective effect of oxygen absorbers on drug products.
5. The permeability of the container
The permeability of the container is a key factor to be considered when selecting packaging materials for products that are sensitive to the respective permeate. The moisture and oxygen transmission rates of packaging materials need to be tested and reported as moisture vapor/water vapor transmission rate (MVTR or WVTR) and oxygen transmission rate (OTR). Table 1 lists the MVTR and OTR data of some packaging materials. It is important to note that the data listed for the blister material in the table refers to a flat film. Due to the reduced thickness of the forming cavity, the permeation rate of the blister will be greater than that of the flat film. The actual dimensions of any given container, including surface area and thickness, should be considered in package design and evaluation.
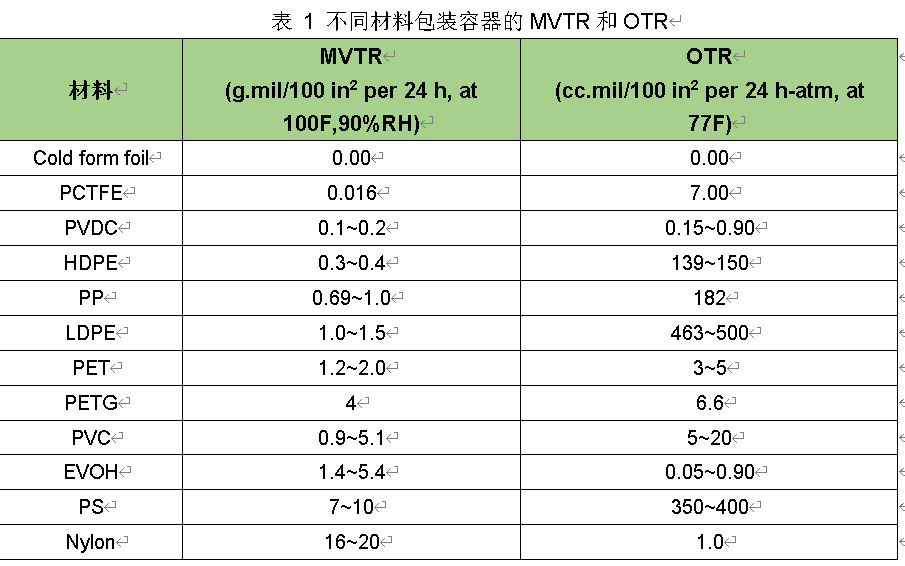
The data in Table 1 shows that materials with low MVTR may not have low OTR. For example, the MVTR of nylon is approximately 1000 times greater than that of PCTFE. However, the OTR of nylon is actually smaller than that of PCTFE. The combination of PCTFE and EVOH can produce multilayer blister materials with excellent moisture and oxygen barrier properties.
While aluminum foil may be considered the ultimate gas barrier, thin foils (thicker than 25 microns) may have puncture holes, and the foil may pass through through these puncture holes. Puncture holes can be caused by the presence of organic contaminants in the molten aluminum or by rolling stress during foil production. The number of perforated holes increases with decreasing foil thickness. This property is an important factor to consider when selecting foil materials and designing packages that use thin foil as a barrier.
5.1 Selection of packaging according to the nature of the product and the permeability of the container
Generally, claims for protection are established during the early development stages using an API or drug product through accelerated degradation studies or forced degradation studies.
If accelerated studies show that the product is not sensitive to moisture and/or other gases, then the protection requirements for the product primarily include protection against contamination and physical stress. If the product is light-sensitive, containers with low light transmission should be preferred, otherwise, if transparent primary packaging is used, secondary packaging should be used to protect it from light. If the product is moisture sensitive, a container with a low MVTR value should be preferred and a desiccant can be used for additional protection.
Source from 药事纵横




没有评论:
发表评论