Pet nutrition cream is a quick-replenishing nutritional supplement, which is mainly used for various nutritional supplements of pets. The pet nutrition cream syringe is a packaging form of this supplement, which is different from ordinary pharmaceutical packaging, and has the function of drug storage and administration at the same time. So, what are the advantages of this special packaging?
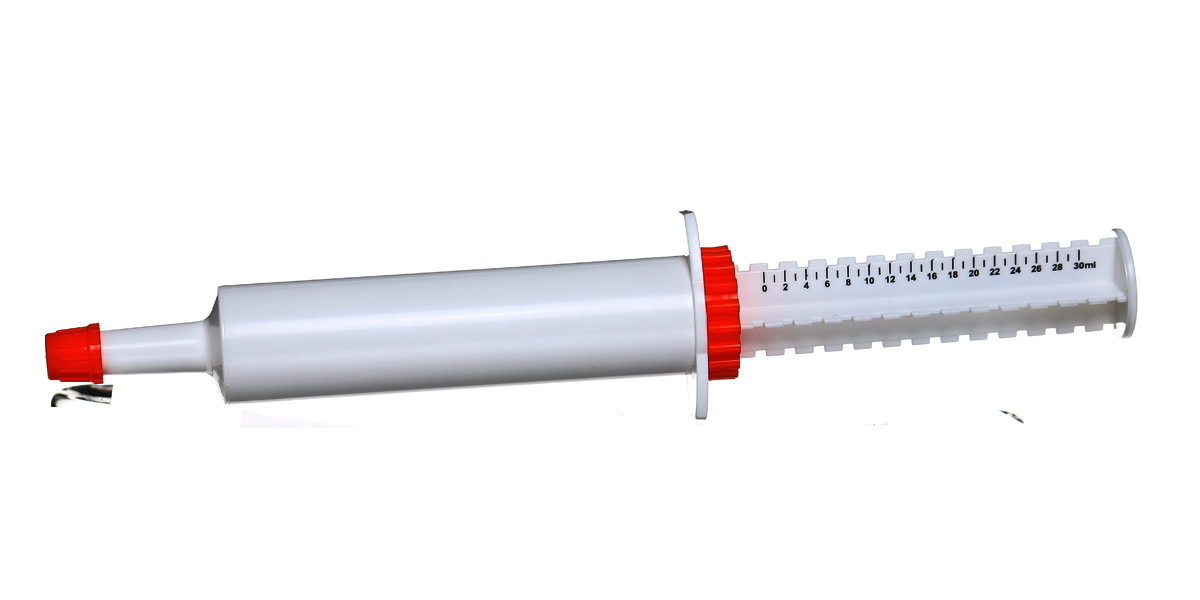
30ml oral dose syringe for dog
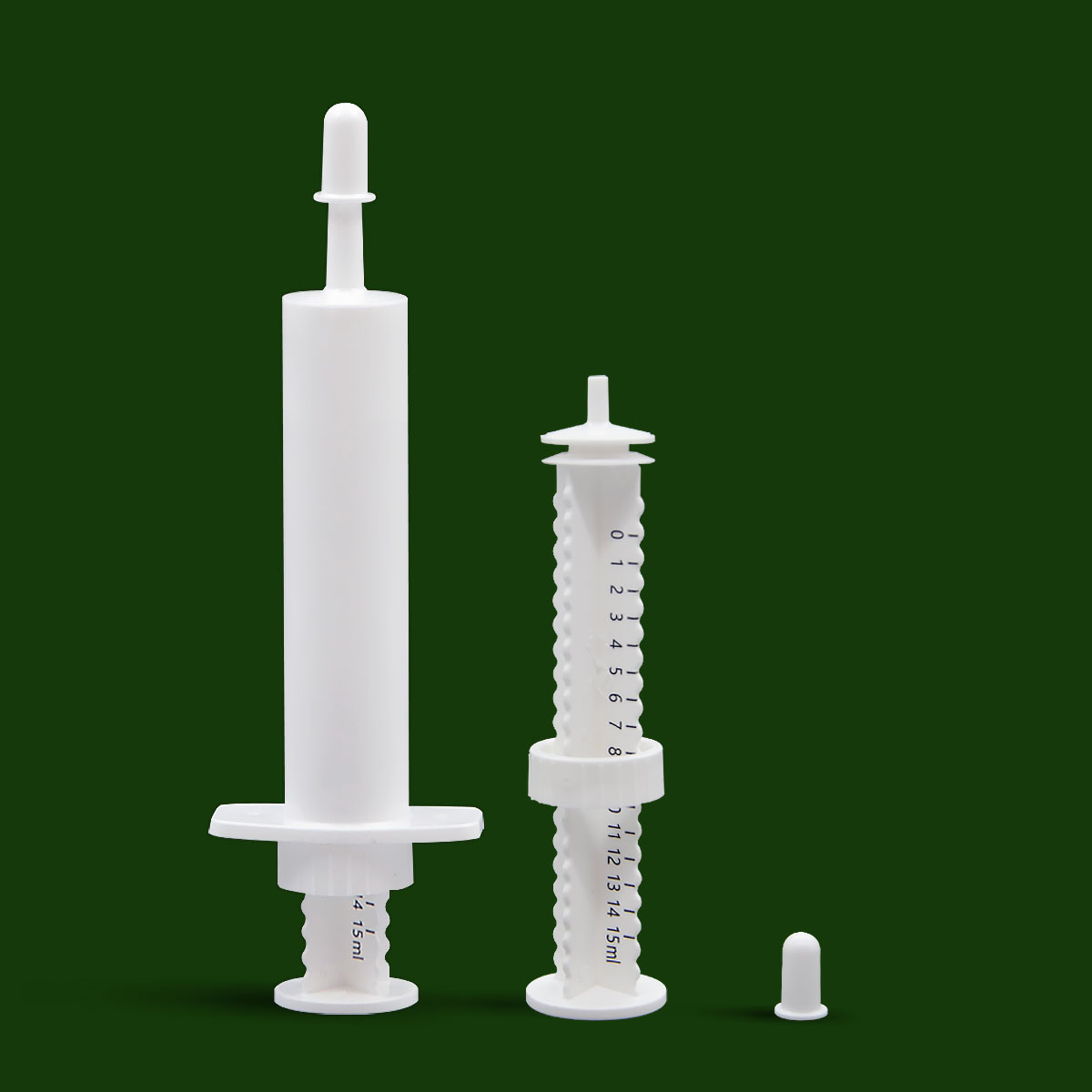
1. Easy to use: The pet nutrition cream syringe is composed of a tube sleeve, a push rod, a piston, a protective cap and a positioning ring. When the protective cap is closed, it is a drug storage container. turned into a drug delivery tool. This package is very convenient to use, remove the protective cap, hold the push rod and handle part with your hand, and gently push the push rod to complete the drug delivery.
2. Quantitative feeding: As a supplement, pet nutritional cream also has certain requirements for feeding dose. Excessive administration can easily lead to excessive nutrition for pets, resulting in problems such as excessive obesity, which is not conducive to the health of pets. The feeding dose is insufficient, and the nutritional supplement effect cannot be achieved. The pet nutrition cream infuser has the function of quantitative feeding. There is a standard scale printed on the push rod. Slide the positioning ring to the corresponding scale, push the push rod, and stop when it reaches the positioning ring, so that the effect of precise dose control can be achieved. .
The pet nutrition cream syringe has two advantages of convenient use and quantitative feeding. This packaging is also widely used in the packaging of cow mastitis drugs, horse oral paste, cockroach medicine and other drugs.