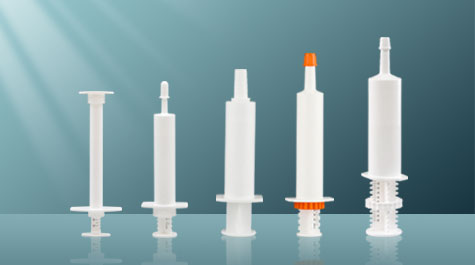
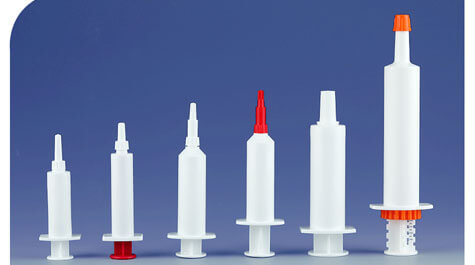
As a health care product, pet nutrition cream has a significant effect on weak, elderly dogs and cats, dogs and cats recovering their physical fitness after surgery and illness, and pets who need to improve immunity. At present, pet nutrition cream has adopted a new type of pre-filled packaging. To ensure the maximum effect of the nutrition cream, the tightness of the package is very important.
Tightness is an important indicator of veterinary packaging quality, related to the product quality and expiration date of the packaging contents, and is also a necessary guarantee for the product circulation environment. As a new type of drug packaging method, prefilling has the characteristics of convenient use and simple operation. Generally speaking, its tightness is mainly controlled by the following points:
First, the sealing member. The use of sealing components that comply with internationally accepted standards is the basis for ensuring the tightness of the package;
Second, the necessary verification. When the components of the sealing system or the potting system are changed, the impact on the sealing system should be evaluated in time, and the sealing integrity should be verified again when necessary;
Third, meet the test requirements. The verification of tightness requirements shall meet the requirements of bacteria intrusion test and saturated salt water test;
Fourth, other experimental evidence. In addition to the conventional experimental methods for prefilled syringes, influencing factor experiments and accelerated experiments should also be carried out to ensure that its sealing performance during the validity period meets user requirements.
The airtightness of the veterinary syringe is of great significance to the efficacy of pet nutrition cream. If the airtightness of the packaging is not good, it will have a certain impact on the brand image of the pharmaceutical company. Therefore, when choosing a packaging manufacturer, the pharmaceutical company should do the packaging performance Comprehensive assessment.